click to see full size image(402Kb)


|
Oxygen gradients exist in normal and tumor tissue. These gradients affect gene expression and are important in normal and pathological conditions. Until recently it was difficult to measure these gradients at the cell level but the advent of 2-nitroimidazole hypoxia markers has made this possible. There are a number of ways that the hypoxia markers can be detected. The immunohistochemical technique is particularly attractive because gradients of hypoxia can be visualized and compared with underlying hierarchical structures in tissues and with gene expession on a cell by cell basis. This website describes the Hypoxyprobe system of 2-nitroimidazole hypoxia markers and its application to studies of tissue hypoxia under both normal and pathological conditions. The hypoxia marker that has received most attention in the Hypoxyprobe system of markers is Hypoxyprobe which is also known as pimonidazole (pee’-mah-nie’-dah-zole’) hydrochloride.
 |
In 1976, Varghese et al. reported that 14C-labelled misonidazole formed adducts in hypoxic cells in vitro and in vivo(1). It was subsequently found that adducts form with thiol groups in proteins, peptides and amino acids in a way that all atoms of the ring and side-chain of the 2-nitroimidazole are retained (2-5). Hypoxia (pO2 < 10 mmHg) is required for binding but binding is not dependent on the presence of specialized redox enzymes such as P450 nitroreductases. Furthermore, wide variations in NADH and NADPH levels do not change the oxygen dependence of binding (6, 7).
Chapman et al. showed that the oxygen dependence of binding was fortuitously close to that for radiation resistance and suggested that misonidazole binding might be used as a hypoxia marker in solid tumors (8). The clinical feasibility of the hypoxia marker idea was demonstrated by means of autoradiographic analyses of 3H-misonidazole binding in a variety of human tumors (9). While the 3H-misonidazole approach had limited clinical utility, it spurred the development of a variety of non-invasive assays for tissue hypoxia based on 2-nitroimidazoles. These included single photon electron capture tomography, positron emission tomography, nuclear medicine analysis and magnetic resonance spectroscopy of suitably labeled 2-nitroimidazole analogues (for review see ref. (10)).
During 19F MRS investigations of tumor hypoxia with the hexafluorinated 2-nitroimidazole, CCI-103F, it became clear that an histological assessment of hypoxia would be a useful complement to non-invasive assays (11-13). This led to the invention of the immunochemical hypoxia marker technique based on monoclonal and polyclonal antibodies raised against protein adducts of reductively activated 2-nitroimidazoles (14, 15). Preclinical testing of immunochemical reagents in spontaneous canine tumors showed that immunochemical hypoxia markers would be useful in their own right (16-21). In addition to providing a quantitative measure of hypoxia, immunohistochemical markers provide insights into microregional relationships between hypoxia and factors such as necrosis, proliferation, differentiation, apoptosis, and oxygen regulated protein expression. A variety of immunochemical hypoxia markers has now been used in clinical (22-27) and preclinical studies (16, 28-33) of such relationships. An example of the unique value of the immunohistochemical marker approach is the observation that neither metallothionein nor vascular endothelial growth factor are expressed in the majority of hypoxic cells in human squamous cell carcinomas (24, 25) even though in vitro studies would have predicted otherwise (34, 35).
With respect to quantifying hypoxia by the immunochemical technique, image analysis (24-27) or flow cytometric analysis (36, 37) appear most promising. Preclinical studies of sampling error showed that stratification of patients is feasible with the immunohistochemical approach if 3-4 biopsies are obtained from geographically separate regions of each tumor. Precision can be increased by increasing the number of sections analyzed per biopsy from 1 to 3 but analysis of multiple biopsies is the most important factor. Interestingly, the accuracy of the immunochemical analysis increases as the amount of hypoxia decreases (19, 21).
The rationale for developing pimonidazole hydrochloride (Hypoxyprobe) as a hypoxia marker for experimental and clinical use was based on its chemical stability, water solubility, wide tissue distribution and, in the case of clinical studies, the fact that human toxicity data were available from earlier radiosensitizer trials. This facilitated early clinical application and Hypoxyprobe kits have now been used in many experimental studies and clinical trials worldwide. Solid Hypoxyprobe is very stable being unchanged after storage for 2 years at room temperature in subdued light. Saline solutions of Hypoxyprobe used for clinical studies (34 millimolar in 0.9% saline, pH 3.9 ± 0.1) are extremely stable being unchanged after 1.5 years at 4oC in subdued light as determined by high performance liquid chromatography and ultraviolet spectroscopy. In addition to chemical stability, Hypoxyprobe has high water solubility (400 millimolar; 116 grams per 1000 mL) that facilitates intravenous marker infusion and produces a short plasma half-life of 5.1 ± 0.8 hours. In spite of the water solubility of its hydrochloride salt (pKa 8.7), pimonidazole itself has an octanol-water partition coefficient of 8.5 (38) and diffuses readily into tumors and normal tissues including brain (39). Consistent with a large, 155 liter volume of distribution, pimonidazole concentrates approximately 3 fold above plasma levels in tumors and normal tissues (39) thereby increasing the sensitivity of hypoxia marking. At the Hypoxyprobe dose of 0.5 g/m2 used in hypoxia marking, pimonidazole causes neither central nervous system toxicity nor sensation (e.g., flushing) (39). Central nervous system toxicity was of particular interest because this was the dose limiting toxicity for Hypoxyprobe at the higher, multiple doses used in radiosensitizer trials. In addition to the absence of central nervous system effects, the overall procedure from Hypoxyprobe infusion to tumor biopsy is well-tolerated in both inpatient and outpatient settings.
Protein adducts of reductively-activated pimonidazole are effective immunogens for the production of both polyclonal and monoclonal antibodies. The antibodies have been used for immunoperoxidase analysis of formalin fixed, paraffin embedded sections (6, 16, 23-26, 32, 40); for immunofluorescence analysis of frozen fixed sections (27, 41-43); and, for flow cytometry with directly labeled or secondary fluorescent antibodies (36). The antibodies have also been used in enzyme linked immunosorbent assays (6, 16, 40). As is the case for Hypoxyprobe itself, the antibodies to pimonidazole adducts are very robust. For example, aqueous solutions of the IgG1 monoclonal antibody against pimonidazole adducts (clone 4.3.11.3) is stable indefinitely when stored at -20oC and is stable for at least 4 months at 4oC when supplemented with 10 mg/mL of bovine serum albumin and 10 millimolar sodium azide. One final attractive feature of pimonidazole is the fact that pimonidazole adducts in vivo are long-lived (16). This provides flexibility in the timing of biopsy taking which is an advantage in a clinical setting. In summary Hypoxyprobe and associated antibodies form a very attractive basis on which to develop a low tech, low cost kit for measuring normal and tumor tissue hypoxia.
Hypoxyprobe kits consist of two parts.
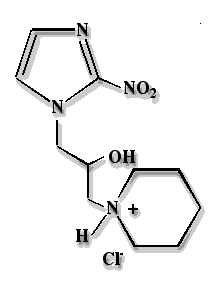
Hypoxyprobe has a molecular weight of 290.7; a water solubility of 116 mg/mL which is equivalent to 400 millimolar and an ultraviolet absorbance maximum at 324 nm with an extinction coefficient of 7400 (free base) in 0.9% saline. The free base, pimonidazole, has a molecular weight of 254.3, a pKa of 8.7 and an octanol water partition coefficient of 8.5. At the concentration of 34 millimolar in 0.9% saline that is used for clinical application, Hypoxyprobe solutions have a pH of 3.9 ± 0.1. Hypoxyprobe has great chemical stability in solid form and in aqueous solutions and requires no stabilizer. For example, solid Hypoxyprobe has been stored for two years at room temperature in subdued light without detectable degradation as assessed by UV and HPLC analyses. Hypoxyprobe solutions in 0.9% saline have been stored at a concentration of 10 gms/liter (34 millimolar) at 4oC in subdued light for 1.5 years without detectable degradation (UV and HPLC analyses). When exposed to laboratory light, Hypoxyprobe slowly turns yellow.
Although doses of Hypoxyprobe up to 400 mg/kg have been used without measurable toxicity in mice (36), a dose of 60 mg/kg body weight is routinely used in studies of tissue hypoxia in rodents. The high water solubility of Hypoxyprobe permits small volume injections to be made which is convenient for studies with small animals. Intravenous injection or intraperitoneal injection can be used. The plasma half-life of Hypoxyprobe is 0.5 hours in C3H/He mice. Hypoxyprobe is distributed to all tissues in the body including brain but binds only to cells that have oxygen concentrations less than 14 micromolar which is equivalent to a pO2 of 10 mm Hg at 37oC. Tumors and normal liver, kidney and skin have cells at, or below, this pO2. For dogs, whole body doses of 0.28 gm/m2 are recommended (16). The plasma half-life for Hypoxyprobe in dogs is 1.5 ± 1.0 hours.
In addition to animal studies, Hypoxyprobe kits can be used for cells in tissue culture(6, 44). Typically, cell suspensions are incubated under hypoxia for 1 to 2 hours in the presence of 100 to 200 micromolar Hypoxyprobe. The cells are harvested by cytospin, fixed and immunostained with an antibody for pimonidazole adducts. Sufficient concentrations of pimonidazole adducts are formed on the surface of cells to elicit a response to complement or activated cytotoxic lymphocytes (44).
Storage. Exhausted hybridoma supernatant containing Hypoxyprobe MAb1 has been stored at -20oC for 6 years without detectable loss of activity. For short term storage, undiluted Hypoxyprobe MAb1 supernatant containing 1 drop/mL of protein block (DAKO) or 10 mg/mL of bovine serum albumin and 10 millimolar sodium azide can be stored at 4oC for up to 4 months without detectable loss of activity.