| Q: What is the best solvent for Hypoxyprobe?
|
| Q. What dose of Hypoxyprobe should be used for hypoxia
marking experiments?
|
| Q. Does Hypoxyprobe penetrate hypoxic brain and brain
tumor tissue?
|
| Q. Is Hypoxyprobe the best probe for detecting hypoxia
in vivo?
|
| Q. Can the monoclonal antibody to Hypoxyprobe adducts be
used on mouse tissue?
|
| Q. What is the mechanism for the activation and binding of
Hypoxyprobe to hypoxic cells?
|
| Q. What is the concentration of the IgG1 monoclonal
antibody in the antibody solution supplied with the Hypoxyprobe kits?
|
| Q. How soon after Hypoxyprobe injection can
tissue be harvested for hypoxia analysis?
|
| Q: Does Hypoxyprobe detect both chronic and acute hypoxia?
|
| Q. On what basis is a threshold of tissue pO2 ≤ 10 mmHg set for Hypoxyprobe
binding?
|
| Q. Where can I find information about Hypoxyprobe (pimonidazole) pharmacokinetics in mice?
|
| Q. Does in vivo oxidation of Hypoxyprobe (pimonidazole) compromise its effectiveness as a hypoxia marker?
|
| Q. What can I do when Hypoxyprobe staining patterns do not appear to be consistent with tissue hypoxia?
|
|
|
| Q: What is the best solvent for Hypoxyprobe?
|
|---|
| A: Hypoxyprobe is the hydrochloride salt of a weak base and, as such, is very soluble in aqueous solutions including neutral buffered saline (116 mg/mL or 400 millimolar). This permits the use of small volume (0.1 mL), intraperitoneal or intravenous injections of Hypoxyprobe in animal studies. |
|
back to top
|
| Q. What dose of Hypoxyprobe should be used for hypoxia
marking experiments? |
| A:The extent of Hypoxprobe binding in hypoxic tissue will depend
on the rate of bioreductive activation and on tissue exposure to Hypoxprobe
(exposure index = concentration x time). It is hard to predict the rate of
reductive metabolism but the effect of exposure can be examined. The calculation
of Hypoxprobe concentration is on the basis of pimonidazole hydrochloride (MW 290.7).
Very good labeling is observed in spheroids exposed in vitro to 58mg/kg (concentration in medium) for 1 hour. The exposure index is 58mg/kg x 1 hour = 58mg/kg-hour. Very good labeling of tumor tissue and normal epithelia is obtained in humans with 0.5gm/m2 or ca 14mg/kg where the plasma half-life of Hypoxprobe is 5 hours. The exposure index is 14 mg/kg x 5 hours = 70 mg/kg-hour. Very good labeling of tumor tissues in mice is obtained with 60 mg/kg where the half-life of Hypoxprobe is 0.5 hours. The exposure index is 60mg/kg x 0.5 hours = 30 mg/kg - hour. In summary, for small animals of uniform size such as laboratory rats and mice, a dose of Hypoxprobe of 60 mg/kg body weight is recommended although doses ranging from 30 mg/kg (47) to 400 mg/kg (36) are effective in mice and rats without toxicity or altered oxygen levels due to blood flow effects. Blood flow effects have been observed at doses above 100 mg/kg of Hypoxyprobe for tumors implanted in the hind legs of mice and caution must be taken, therefore, when doses > 100 mg/kg are used in this model. For larger animals with non-uniform body size, the dose is typically calculated on the basis of surface area. For humans, the recommended dose is 0.5 gm/m2 (23) while for dogs a dose of 0.28 gm/m2 has been used (16). |
|
back to top
|
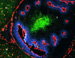
| Q. Does Hypoxyprobe penetrate hypoxic brain and brain
tumor tissue? |
| A: Although Hypoxyprobe is water soluble, its corresponding free base has an octanol water coefficient of 8.5 and, as a result, the marker freely penetrates into both brain and brain tumor tissue (48, 49). |
| Photomicrograph of a E106 human gliomablastoma multiforme xenograft growing in the brain of a nude mouse. |
|---|
| click to see full size image(244Kb) |
 |
| Immunofluorescence staining of the frozen section reveals vasculature (ME 9F1; red) and hypoxia (Hypoxyprobe adducts; green) in combination with perfusion (Hoechst 33342;(blue) in normal brain (N) and tumor tissue (T). Original magnification x 200. Note the regular pattern of vasculature in normal brain versus poorly organized vasculature in tumor tissue (Bernsen et al, Journal of Neurosurgery 93: 449-454, 2000; by permission)(43). |
|
back to top
|
| Q. Is Hypoxyprobe the best probe for detecting hypoxia
in vivo? |
|---|
| A: Hypoxyprobe has some real advantages as a hypoxia marker.
Foremost is its high solubility in aqueous solution (400 millimolar; 116 mg/mL of saline) which
allows the marker to be administered to rodents as small volume
injections of saline solutions (0.1mL) either intraperitoneally or intravenously.
Markers such as the hexafluorinated CCI-103F have aqueous solubilities of
10 millimolar or less and are usually administered as intraperitoneal emulsions
of peanut oil and DMSO in order to avoid hemodilution (45).
Although Hypoxyprobe, the hydrochloride salt of pimonidazole, is very water soluble, pimonidazole itself has an octanol-water partition coefficient of 8.5 and penetrates all tissues including brain (see above). Another advantage is that pimonidazole binding can be detected by immunofluorescence in frozen fixed tissue sections; by immunoperoxidase in formalin fixed paraffin embedded tissue sections; by ELISA or by flow cytometry. |
|
back to top
|
| Q. Can the monoclonal antibody to Hypoxyprobe adducts be
used on mouse tissue? |
| A: Yes. For formalin fixed paraffin embedded tissues we recommend a peroxidase F(ab)2 secondary antibody strategy (32). This gives a very clean background and is applicable to a variety of animal species. |
|
back to top
|
| Q. What is the mechanism for the activation and binding of
Hypoxyprobe to hypoxic cells? |
| A:Varghese et al. were first to show that hypoxic cells activate
and bind 2-nitroimidazole compounds (1). Subsequent studies by a number of
investigators contributed to an understanding of the overall mechanism of
bioreductive activation and binding of 2-nitroimidazoles (2-7). The current
view of the metabolism of Hypoxyprobe is summarized in the scheme below.
In vivo, Hypoxyprobe is subject to oxidative metabolism leading to easily
excretable N-oxide, sulfate and glucuronate derivatives (ST = sulfotransferase;
GT = glucuronly transferase). These pathways do not appear to interfere
with the utility of Hypoxyprobe at the doses used for hypoxia marking
(7). Oxygen competes for the addition of the first electron to Hypoxyprobe and it is believed that this competition accounts for the oxygen dependence of 2-nitroimidazole reductive activation and binding. The chemical structure of adducts to proteins is not known with certainty but the available evidence supports the idea that the adducts are similar to the 4 and 5 adducts of glutathione to reductively activated 2-nitroimidazoles. It is unlikely that all reductively activated Hypoxyprobe binds to cellular molecules and, on the basis of model studies, it is estimated that ca 80% of reductively activated intermediates such as the hydroxylamine derivative are fragmented by reaction with water (4). |
| Summary of the current view of the metabolism of Hypoxyprobe |
|---|

|
| back to top
|
| Q. What is the concentration of the IgG1 monoclonal
antibody in the antibody solution supplied with the Hypoxyprobe kits?
|
|---|
| A:The concentration of the mouse IgG1 monoclonal antibody is 70 micrograms/mL. |
|
back to top
|
| Q. How soon after Hypoxyprobe injection can
tissue be harvested for hypoxia analysis?
|
|---|
| A:This question arises because tissues can become anoxic during harvesting.
Activation and binding of circulating Hypoxyprobe during this period might,
therefore, give false measures of hypoxia. The answer lies in an examination of overall
tissue exposure to Hypoxyprobe. Overall exposure is defined as marker
concentration multiplied by time of exposure at 37oC. Temperature comes into
play because the biochemically induced binding is temperature dependent.In principle,
a combination of low marker concentration, rapid harvest and immediate fixation in
cold medium will eliminate measurable levels of non-specific binding. In the initial human tumor studies, biopsies were taken 16-24 hours after Hypoxprobe infusion (24).The plasma half-life of Hypoxyprobe-1 in humans is ca 5 hours so that 16 to 24 hours represents 3 to 5 half-lives of circulating marker.This means that 1/8 to 1/32 of the initial concentration of marker is present at the time of harvesting. This, combined with rapid transfer of biopsy material to cold fixative, minimized non-specific Hypoxyprobe binding as shown by low background binding in the majority of cells close to blood vessels. Subsequent studies have shown that long, post infusion harvesting times are not necessary. In recent human tumor studies, biopsies were taken only 1.5 to 4 hours after Hypoxyprobe infusion.The biopsies were immediately fixed in liquid nitrogen (27). This approach gave low background binding in the majority of cells near blood vessels. Although the level of circulating Hypoxyprobe is relatively high 1.5 to 4 hours after infusion, the exposure time at 37oC in harvested tissue was extremely short leading to undetectable levels of non-specific binding. The experience with human tumors can guide experimental studies. The plasma half-life of Hypoxyprobe in mice is typically 0.5 hours.Under these circumstances, a harvest time of 2 hours combined with rapid addition to cold fixative will be effective in eliminating non-specific binding. This approach is particularly appropriate for experiments involving carbon dioxide asphyxiation where the duration of global hypoxia is poorly defined. More rapid euthanasia techniques lend themselves to shorter times of harvest as long as rapid tissue harvest and fixation in cold fixative is carried out. |
|
back to top
|
| Q: Does Hypoxyprobe-1 detect both chronic and acute hypoxia?
|
|---|
| A:Two categories of hypoxia are currently recognized in solid tissues – diffusion-limited
chronic hypoxia and perfusion-limited acute hypoxia. Chronic hypoxia arises at the
distal end of oxygen gradients created by oxygen consumption in cells close to blood
vessels compounded, in the case of tumors, by deficiencies in local oxygen supply arising
from longitudinal gradients of pO2 in vascular trees (54). The presence of chronic
hypoxia implies that cells in tissues consume oxygen at a rate that is independent of
oxygen supply thereby driving pO2 to very low levels in microregions distal to blood
vessels. That is, most cells possess characteristics of a “regulating” cellular phenotype
that is preprogrammed to adapt to low pO2 by smoothly transitioning to glycolytic based
energy production (55). In contrast to chronic hypoxia with static, metabolically controlled pO2 gradients, acute hypoxia is associated with fluctuating pO2 that results from blood flow instabilities which, in the case of tumors, is created by transient vascular occlusion (54). It has been proposed that acutely hypoxic tumor cells, being proliferative, might be more therapeutically relevant (56) than quiescent, chronically hypoxic cells (23, 26). In normal tissues, fluctuating hypoxia is associated with hypoxia-reperfusion injury (57-59). With respect to whether pimonidazole can detect both chronic and acute hypoxia, compounds that incorporate weakly basic substituents (pKa ≥ 8.0) are concentrated in tissues ca 3 fold above circulating blood levels (39, 60). This property of weakly basic compounds is based on the effect of differentials in intra- and extracellular pH on intracellular concentrations of weakly basic compounds. In particular, at pH 7.4 weakly basic 2-nitroimidazoles are concentrated intracellularly 2-fold compared to extracellular concentration (61). This concentration increase is directly reflected in increased hypoxic cell radiosensitization (62) and labeling with hypoxia markers (63). Because cells experiencing fluctuating hypoxia are proximal to blood vessels and at relatively high pH (64), weakly basic, 2-nitroimidazole hypoxic markers such as pimonidazole are concentrated in these cells whereby episodes of acute hypoxia lead to higher levels of binding compared to hypoxia markers lacking weakly basic moieties. In this way, pimonidazole and its analogues are superior for detecting acute hypoxia (see Kleiter et al for further discussion)(63). |
|
back to top
|
| Q. On what basis is a threshold of tissue pO2 ≤ 10 mmHg set for Hypoxyprobe-1
binding?
|
|---|
| A:It is difficult to measure the Km(O2) for nitroimidazole binding in solid tissue. The best
experiment to date is a comparison between oxygen microelectrode measurements of pO2
and misonidazole binding as measured by autoradiography of radioactively labeled
misonidazole in the spheroid model of solid tissue (65). Gross et al found that grain
densities due to misonidazole binding increased steeply below 10 mm Hg. We found that
the Km(O2) for pimonidazole binding is similar to that for misonidazole in HeLa cells (6)
and came to the conclusion that 10 mm Hg is a reasonable threshold value for
pimonidazole binding in solid tissue as well. A feature of solid tissues that is absent in sparse cell cultures is that oxygen consumption creates very steep O2 gradients so that the distance over which very different Km(O2)s are traversed is foreshortened. For example, steep pO2 gradients are observed in liver tissue wherein immunostaining for pimonidazole adducts goes from background to intense staining over a few cell diameters (6). Consistent with the presence of steep pO2 gradients in tissues are the immunostaining patterns for oxygen regulated proteins such as involucrin and carbonic anhydrase IX that closely resemble those for pimonidazole binding even though the Km(O2) for oxygen regulated proteins is ca 15 mm Hg compared to 2-4 mm Hg for pimonidazole binding in vitro (see Chou et al. for discussion)(66). |
|
back to top
|
| Q. Where can I find information about Hypoxyprobe (pimonidazole) pharmacokinetics in mice?
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
A:
| ||||||||||||
|
A. Plasma pharmacokinetics and HPLC conditions. 1. Stratford, M. R., Minchinton, A. I., Hill, S. A., McNally, N. J., and Williams, M. V. Pharmacokinetic studies using multiple administration of RO 03-8799, a 2-nitroimidazole radiosensitizer. Int J Radiat Oncol Biol Phys, 8: 469-471, 1982. 2. Stratford, M. R. L., Minchinton, A. I., Stewart, F. A., and Randhawa, V. S. Pharmacokinetic studies of some novel (2-nitro-1-imidazolyl) propanolamine radiosensitizers. In: A. Breccia, C. Rimondi, and G. E. Adams (eds.), Advanced Topics on Radiosensitization of Hypoxic Cells, pp. 165-169. New York: Plenum, 1982. 3. Williams, M. V., Denekamp, J., Minchinton, A. I., and Stratford, M. R. In vivo assessment of basic 2-nitroimidazole radiosensitizers. Br J Cancer, 46: 127-137, 1982. 4. Williams, M. V., Denekamp, J., Minchinton, A. I., and Stratford, M. R. In vivo testing of a 2-nitroimidazole radiosensitizer (Ro 03-8799) using repeated administration. Int J Radiat Oncol Biol Phys, 8: 477-481, 1982. 5. Malcolm, S. L., Lee, A., and Groves, J. K. High-performance liquid chromatographic analysis of the new hypoxic cell radiosensitiser, Ro 03-8799, in biological samples. J Chromatogr, 273: 327-333, 1983. 6. Walton, M. I., Bleehen, N. M., and Workman, P. The reversible N-oxidation of the nitroimidazole radiosensitizer Ro 03- 8799. Biochem Pharmacol, 34: 3939-3940, 1985. 7. Walton, M. I., Bleehen, N. M., and Workman, P. The effects of whole body hyperthermia on the pharmacokinetics and toxicity of the basic 2-nitroimidazole radiosensitizer Ro 03-8799 in mice. Br J Cancer, 55: 469-476, 1987. 8. Ward, R. and Workman, P. Gradient high-performance liquid chromatographic method for simultaneous assay of the radiosensitizers etanidazole (SR 2508) and pimonidazole (Ro 03-8799) in biological materials. J Chromatogr, 420: 223-227, 1987. 9. Walton, M. I., Bleehen, N. M., and Workman, P. Effects of localised tumour hyperthermia on pimonidazole (Ro 03-8799) pharmacokinetics in mice. Br J Cancer, 59: 667-673, 1989. 10. Workman, P. Accelerated elimination of pimonidazole following microsomal enzyme induction in mice: a possible approach to reduced neurotoxicity of the pimonidazole-etanidazole combination. Int J Radiat Oncol Biol Phys, 16: 1011-1014, 1989. 11. Workman, P., Newman, H. F., Bleehen, N. M., Ward, R., and Smithen, C. E. Lack of stereoselectivity in the pharmacokinetics and metabolism of the radiosensitizer Ro 03-8799 in man. Cancer Chemother Pharmacol, 28: 118-122, 1991. B. Other pharmacokinetic data of interest. 1. Smithen, C., Clarke, E., Dale, J., Jacobs, R., Wardman, P., Watts, M., and Woodstock, M. Novel (nitro-1-imidazoyl)-alkanolamines as potential radiosensitizers with improved therapeutic properties. In: L. W. Brady (ed.), Radiation Sensitizers. Their use in the clinical management of cancer, pp. 22-32. New York: Masson, 1980. 2. Denekamp, J., Michael, B. D., Minchinton, A. I., Smithen, C. E., Stewart, F. A., Stratford, M. R., and Terry, N. H. Comparative studies of hypoxic-cell radiosensitization using artificially hypoxic skin in vivo. Br J Cancer, 45: 247-255, 1982. 3. Hill, S. A., Fowler, J. F., Minchinton, A. I., Stratford, M. R., and Denekamp, J. Radiosensitization of a mouse tumour by Ro 03-8799: acute and protracted administration. Int J Radiat Biol Relat Stud Phys Chem Med, 44: 143-150, 1983. 4. Minchinton, A. I. and Stratford, M. R. A comparison of tumor and normal tissue levels of acidic, basic and neutral 2-nitroimidazole radiosensitizers in mice. Int J Radiat Oncol Biol Phys, 12: 1117-1120, 1986. 5. Stone, H. B., Luu, Y. H., and Lam, K. N. Sensitization by SR-2508 plus Ro 03-8799. Int J Radiat Oncol Biol Phys, 12: 1097-1100, 1986. 6. Tamulevicius, P., Luscher, G., and Streffer, C. Effects on intermediary metabolism in mouse tissues by Ro-03-8799. Br J Cancer, 56: 315-320, 1987. 7. Honess, D. J., Wasserman, T. H., Workman, P., Ward, R., and Bleehen, N. M. Additivity of radiosensitization by the combination of SR 2508 (etanidazole) and Ro 03-8799 (pimonidazole) in a murine tumor system. Int J Radiat Oncol Biol Phys, 15: 671-675, 1988. 8. Lespinasse, F., Ch, T., Bonnay, M., Malaise, E. P., and Guichard, M. Ro 03-8799: Preferential relative uptake in human tumor xenografts compared to a murine tumor: Comparison with SR-2508. Int. J. Radiat. Onc. Biol. Phys., 16: 1105-1109, 1989. 9. Stone, H. B., Hirst, V. K., Cribbs, R., Luu, Y. H., and Brown, J. M. A comparison of radiosensitization by etanidazole and pimonidazole in mouse tumors. Int J Radiat Oncol Biol Phys, 20: 987-995, 1991. | ||||||||||||
|
back to top
|
| Q. Does in vivo oxidation of Hypoxyprobe (pimonidazole) compromise its effectiveness as a hypoxia marker?
|
|---|
| A: The piperidine moiety in pimonidazole is easily oxidized to its N-oxide metabolite that, in principle,
could impact the effectiveness of pimonidazole as a hypoxia marker. (See Arteel et al for a detailed discussion of
the metabolism underlying the use of pimonidazole as a hypoxia marker (1)). Pimonidazole N-oxide is formed in vivo
via the action of flavin mono-oxygenases (FMO). FMO isoform distribution varies among species producing different
plasma levels of N-oxide (2, 3) although the route of pimonidazole administration has little impact on plasma levels
of N-oxide (2). Hydrogen peroxide (4) and other, strong oxidants such as peroxynitrate formed by the reaction of
superoxide anion with nitrous oxide (NO) can oxidize pimonidazole raising the possibility that pimonidazole N-oxide
is produced in regions of fluctuating hypoxia in tumor and normal tissue.
Although pimonidazole N-oxide can be formed by a number of mechanisms in vivo, there is reason to believe that this does not compromise the effectiveness of pimonidazole as a hypoxia marker. First, N-oxide formation is reversible due to the reducing action of heme-iron complexes in blood and reductases such as xanthine dehydrogenase and reduced cytochrome P-450 in tissues whereby the loss of pimonidazole by oxidation is limited (4). Second, it has been shown by the use of a second hypoxia marker, that the extent of hypoxia marking by pimonidazole is independent of pimonidazole plasma concentrations at the concentration recommended for hypoxia marking (2). Third, because there is no cross reactivity between pimonidazole and its N-oxide derivative for anti-pimonidazole antibodies, the N-oxide does not interfere with the detection of pimonidazole adducts in hypoxic tissues (2). 1. Arteel, G. E., Thurman, R. G., and Raleigh, J. A. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem, 253: 743-750, 1998. 2. Kleiter, M. M., Thrall, D. E., Malarkey, D. E., Ji, X., Lee, D. Y., Chou, S. C., and Raleigh, J. A. A comparison of oral and intravenous pimonidazole in canine tumors using intravenous CCI-103F as a control hypoxia marker. Int J Radiat Oncol Biol Phys, 64: 592-602, 2006. 3. Roberts, J. T., Bleehen, N. M., Walton, M. I., and Workman, P. A clinical phase I toxicity study of Ro 03-8799: plasma, urine, tumour and normal brain pharmacokinetics. Br J Radiol, 59: 107-116, 1986. 4. Walton, M. I., Bleehen, N. M., and Workman, P. The reversible N-oxidation of the nitroimidazole radiosensitizer Ro 03- 8799. Biochem Pharmacol, 34: 3939-3940, 1985. |
|
back to top
|
| Q. What can I do when Hypoxyprobe staining patterns do not appear to be consistent with tissue hypoxia?
|
|---|
| A: To date, immunostaining patterns for pimonidazole (Hypoxyprobe) adducts are generally consistent with the presence of tissue hypoxia. However, the British pathologist, Leon Cobb, questioned at one time whether high concentrations of nitroreductase enzymes in the pericentral region of liver, for example, might overwhelm the oxygen inhibition of pimonidazole binding and lead to erroneous conclusions about the presence of hypoxia marker binding around the pericentral vein. We met this important challenge by examining the binding of pimonidazole in the pericentral region of rat livers during normal (anterograde) and reverse (retrograde) perfusion of dissected livers. Reverse perfusion causes liver regions around the pericentral vein to become oxygenated and those around the portal vein to become hypoxic. If high concentrations of redox enzymes in the pericentral region caused pimoindazole binding in the presence of oxygen then we would expect to see high levels of pimonidazole binding in the pericentral region during reverse perfusion. In fact, reverse perfusion shifted pimonidazole binding from the oxygenated pericentral region to the now hypoxic periportal region (1) making it clear that high concentrations of cytochrome redox enzymes in the pericentral region, per se, do not obviate oxygen inhibition of pimonidazole binding. An interesting exception to this generalization was a single layer of cells around the pericentral vein. The biochemistry that leads to pimonidazole binding in these cells in the presence of oxygen remains a mystery. It might be that they possess nitroreductases that effectively transfer reducing equivalents (electrons, hydride ions) to nitroimidazole hypoxia markers in two electron steps in way that molecular oxygen cannot intercept them. “Diaphorase” is one such enzyme and investigators must be aware of this caveat with respect to pimonidazole binding when venturing into new territory. It is conceivable, for example, that inflammatory cells can bind pimonidazole in the absence of tissue hypoxia. First, inflammatory cells can possess nitroreductases that reduce pimonidazole in an oxygen insensitive manner. In addition, inflammatory cells can consume oxygen at rates high enough to generate intracellular hypoxia. Individual inflammatory cells labeled in a sea of unlabeled cells should certainly raise concerns about whether the labeled cells are hypoxic.
In analogy to Cobb’s concern, some investigators have asked whether high concentrations of NADH and NADPH in cells driven into in a reduced state might obviate oxygen inhibition of pimonidazole binding. The answer is again, no, at the concentrations of pimonidazole recommended for use in hypoxia marking studies (2). Generally speaking, when the mechanism of pimonidazole binding comes into question, it is useful to be able to intentionally manipulate hypoxia in tissues of interest. One technique is to have animals breathe 10% oxygen during pimonidazole exposure. If the extent of pimonidazole binding increases over that seen in air breathing animals then it is likely that hypoxia is the cause of pimonidazole binding. Parliament and colleagues exploited this approach in studies of tissue hypoxia with misonidazole, a closely related analogue of pimonidazole (3). Using a “diaphorase” inhibitor they demonstrated that none of the binding of misonidazole was due to the presence of the oxygen insensitive, “diaphorase” type nitroreductases (3). Similarly, a good correlation between pimonidazole binding and oxygen microelectrode measurements (r2 = 0.85) was observed when tumor hypoxia was intentionally manipulated by the use of oxygen breathing, carbogen breathing, hydralazine injection or tissue clamping (4). From a technical point of view, it is possible to use a double marking approach to detect changes in a single tissue before and after an intervention that alters tissue pO2. Van der Kogel and his group in Nijmegen have used this approach in animal tumors using the two markers, Hypoxyprobe-1 (pimonidazole HCl) and Hypoxyprobe-F6 (CCI-103F) (5-7). 1. Arteel, G. E., Thurman, R. G., Yates, J. M., and Raleigh, J. A. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer, 72: 889-895, 1995. 2. Arteel, G. E., Thurman, R. G., and Raleigh, J. A. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur. J. Biochem., 253: 743-750, 1998. 3. Parliament, M. B., Wiebe, L. I., and Franko, A. J. Nitroimidazole adducts as markers for tissue hypoxia: mechanistic studies in aerobic normal tissues and tumour cells. Br. J. Cancer, 66: 1103-1108, 1992. 4. Raleigh, J. A., Chou, S. C., Arteel, G. E., and Horsman, M. R. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumors. Radiat Res, 151: 580-589, 1999. 5. Ljungkvist, A. S., Bussink, J., Rijken, P. F., Raleigh, J. A., Denekamp, J., and Van Der Kogel, A. J. Changes in tumor hypoxia measured with a double hypoxic marker technique. Int J Radiat Oncol Biol Phys, 48: 1529-1538, 2000. 6. Ljungkvist, A. S., Bussink, J., Kaanders, J. H., Rijken, P. F., Begg, A. C., Raleigh, J. A., and van der Kogel, A. J. Hypoxic cell turnover in different solid tumor lines. Int J Radiat Oncol Biol Phys, 62: 1157-1168, 2005. 7. Ljungkvist, A. S., Bussink, J., Kaanders, J. H., and van der Kogel, A. J. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res, 167: 127-145, 2007. |
|
back to top
|